Sakamakon mahadi na mercury
Metallic Mercury da mahadinsa suna da matuƙar guba ga rayayyun halittu. Wannan gaskiya ne musamman ga mahadi masu narkewa sosai a cikin ruwa. Dole ne a kula sosai yayin gwaji tare da haɗuwa da wannan sinadari na musamman (mercury shine kawai ƙarfe wanda yake da ruwa a cikin ɗaki). Yarda da ainihin ƙa'idodin likitancin sunadarai? zai ba ka damar gudanar da gwaje-gwaje da yawa cikin aminci tare da mahadi na mercury.
A cikin gwaji na farko, mun sami aluminium amalgam (maganin wannan ƙarfe a cikin mercury ruwa). Maganin Mercury (II) Hg nitrate (V) Hg (NO3)2 da guntun waya na aluminium (hoto na 1). Ana sanya sandar aluminium (a tsantsa da tsaftar adibas) a cikin bututun gwaji tare da maganin gishirin mercury mai narkewa (hoto 2). Bayan wani lokaci, za mu iya lura da sakin gas kumfa daga saman waya (hotuna 3 da 4). Bayan cire sandar daga maganin, sai ya juya cewa yumbu yana rufe da murfin mai laushi, kuma a Bugu da kari, muna kuma ganin bukukuwa na mercury na ƙarfe (hotuna 5 da 6).
Chemistry - gwanintar hada mercury
A ƙarƙashin yanayi na al'ada, an lulluɓe saman aluminum tare da madaidaicin Layer na aluminum oxide.2O3yadda ya kamata ya ware karfe daga mummunan tasirin muhalli. Bayan tsaftacewa da nutsar da sanda a cikin maganin gishiri na mercury, Hg ions suna gudun hijira2+ mafi aiki aluminum
Mercury da aka ajiye a saman sandar ya samar da wata al’ada mai dauke da aluminum, wanda hakan ke sa ya yi wahala ga sinadarin Oxide ya manne da shi. Aluminum karfe ne mai aiki sosai (yana amsawa da ruwa don sakin hydrogen - ana lura da kumfa gas), kuma ana iya amfani da shi azaman kayan aiki mai yuwuwa saboda murfin oxide mai yawa.
A gwaji na biyu, za mu gano ammonium NH ions.4+ ta amfani da Nessler's reagent (masanin ilmin sunadarai na Jamus Julius Nessler shine farkon wanda ya fara amfani da shi a cikin bincike a cikin 1856).
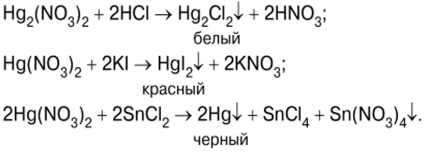
Gwaji kan halayen hops da mercury mahadi
Gwajin yana farawa da hazo na mercury(II) iodide HgI.2, bayan hadawa mafita na potassium iodide KI da mercury (II) nitrate (V) Hg (NO)3)2 (Hoto na 7):
Ruwan lemu-ja na HgI2 (hoto na 8) sannan a bi da shi tare da wuce haddi na potassium iodide maganin don samun hadadden fili mai narkewa na dabara K2HgI4 ? Potassium tetraiodercurate (II) (Hoto 9), wanda shine Reagent Nessler:
Tare da abin da ya haifar, zamu iya gano ions ammonium. Har ila yau za a buƙaci mafita na sodium hydroxide NaOH da ammonium chloride NH.4Cl (hoto na 10). Bayan ƙara ƙaramin adadin gishirin ammonium zuwa Nessler reagent da alkalizing matsakaici tare da tushe mai ƙarfi, muna lura da samuwar launin rawaya-orange na abun ciki na gwajin gwajin. Ana iya rubuta martanin halin yanzu kamar:
Sakamakon sinadarin mercury yana da hadadden tsari:
Ana amfani da gwajin Nessler mai mahimmanci don gano ko da alamun gishirin ammonium ko ammonia a cikin ruwa (misali ruwan famfo).

