Akwai ci gaba a fasahar batirin Li-S: sama da 99%. ikon bayan 200 hawan keke
Masana kimiyya a Jami'ar Melbourne (Australia) sun ba da sanarwar ci gaba a fasahar daidaita batirin lithium-sulfur (Li-S). Sun sami damar ƙirƙirar sel waɗanda ke riƙe sama da kashi 99 na ƙarfinsu bayan zagayowar aiki 200 kuma sun ba da damar yawan ƙwayoyin lithium-ion don nauyi ɗaya.
Abubuwan Li-S - akwai matsaloli, akwai mafita
Tunanin yin amfani da sulfur a cikin sel ba sabon abu bane: An riga an yi amfani da batir Li-S a cikin 2008 akan Zephyr-6, wanda ya karya rikodin rikodi na kewayon da ba saukowa ba. Zai iya kasancewa cikin iska na kusan kwanaki 3,5 godiya ga batura lithium-sulfur masu nauyi waɗanda suka kunna injin kuma suka caje kansu daga batir na hotovoltaic (source).
Koyaya, ƙwayoyin Li-S suna da babban koma baya: jure har zuwa dubun dubatar aikiDomin lokacin da ake caji, cathode da aka yi da sulfur yana ƙaruwa da kusan kashi 78 cikin ɗari (!), wanda ya ninka na graphite sau 8 a cikin ƙwayoyin lithium-ion. Kumburi na cathode yana sa shi ya rushe kuma ya narke sulfur a cikin electrolyte.
Kuma ƙananan girman cathode, ƙananan ƙarfin dukan tantanin halitta - lalata yana faruwa nan da nan.
> Yaya tsawon lokacin da motar lantarki zata kasance? Shekaru nawa ne baturin ma'aikacin lantarki ke maye gurbin? [ZAMU AMSA]
Masana kimiyya na Melbourne sun yanke shawarar manna kwayoyin sulfur tare da polymer, amma sun ba su ɗan sarari fiye da da. An maye gurbin wani ɓangare na m shaidu da gadoji na polymer mai sassauƙa, wanda ya ba da damar samun babban juriya ga lalata tare da canjin ƙarar - gadoji suna manne abubuwan cathode kamar roba:
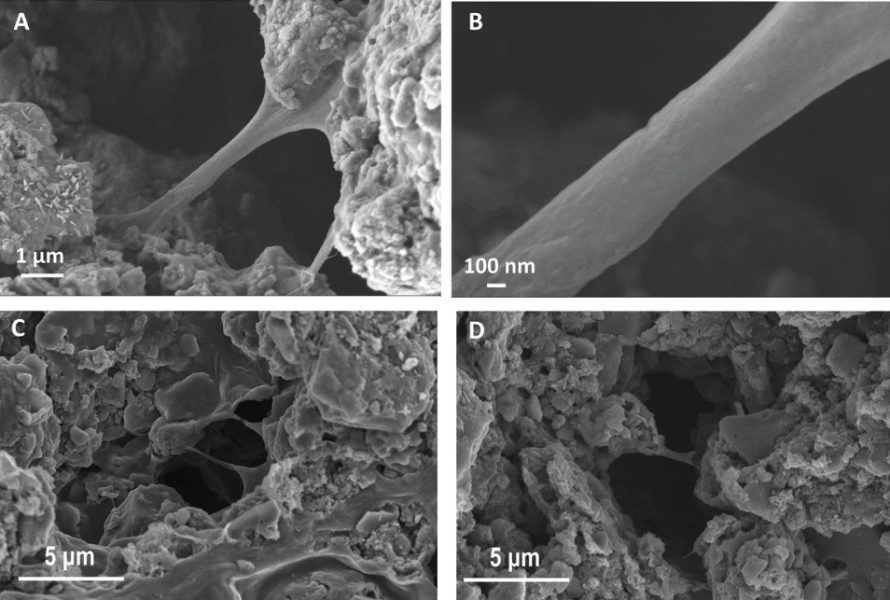
Polymer gadoji masu haɗa tsarin kwayoyin sulfur (c) Jami'ar Melbourne
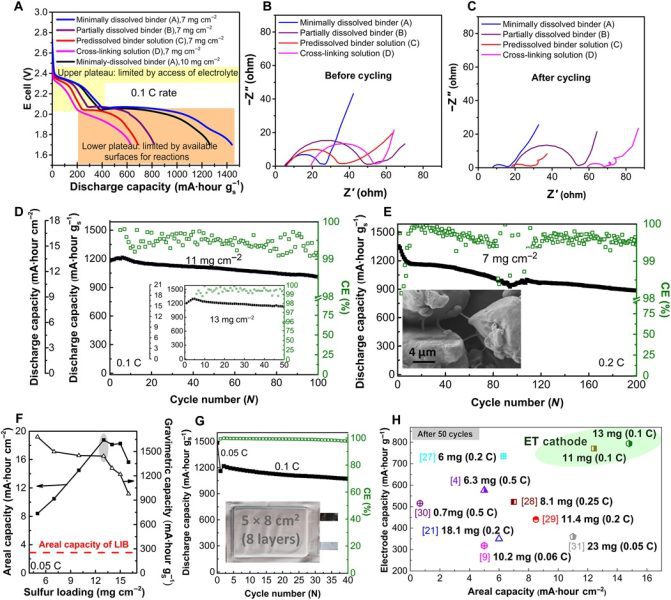
Kwayoyin da irin waɗannan ingantattun cathodes suna da mafi kyawun su. sun sami damar kula da kashi 99 na iyawar su na asali bayan fiye da 200 na hawan keke (madogara). Kuma sun riƙe babbar fa'idar sulfur: suna adana kuzari har sau 5 a kowace juzu'i fiye da ƙwayoyin lithium-ion.
Minuses? Caji da fitarwa sun faru a ƙarfin 0,1 C (ikon 0,1 x), bayan sake zagayowar 200, har ma da mafi kyawun mafita sun ragu zuwa kashi 80 na ƙarfinsu na asali... Bugu da kari, a mafi girma lodi (caji / fitarwa a 0,5 C), kwayoyin sun rasa kashi 20 cikin dari na karfin su bayan dozin da yawa, zuwa matsakaicin sama da 100 na hawan keke.
Hoton buɗewa: Oxis lithium-sulfur cell, wanda ke da nufin yin kasuwanci da wannan fasaha. Hoto mai kwatanta
Wannan na iya sha'awar ku:
